Substance and product data management services
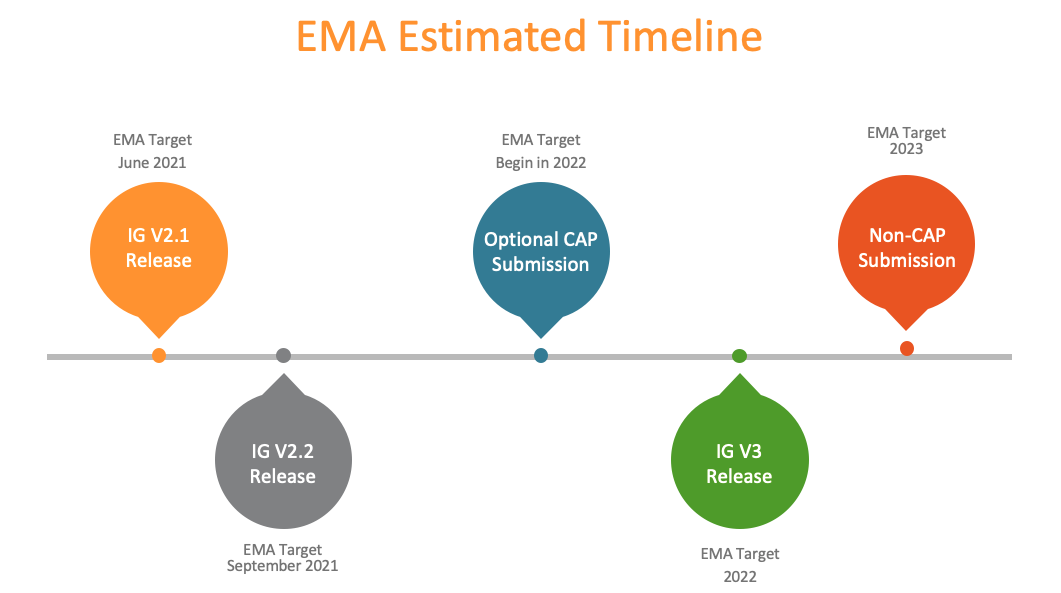
EMA released version 2.1 of the EU IDMP Implementation Guide (EU IG v2.1) in June 2021.
EU IG v2.1 is a minor release of version two of the guide, available since February 2021 as EU IG v2.0, which supports the implementation of PMS Step 1 and data submission on medicinal products authorised under the centralised procedure.
EMA is providing minor releases of version two during 2021 to reflect the latest agreements and details available.
The updates in EU IG v2.1 focus on enhancing data-related aspects of the guide. A forthcoming minor release (EU IG v2.2) will focus mostly on process clarifications for the centralised procedure.
EU IG v3 will support the implementation of PMS Step 2 for other EU authorization procedures and reflect the latest agreements and details available.
EMA first delivered version one (EU IG v1) in February 2020 to help stakeholders plan and prepare for the implementation of ISO IDMP standards.
The guide is composed of following chapters:
- Chapter 1 – Registration requirements
- Chapter 2 – Data elements for the electronic submission of information on medicinal products for human use
- Chapter 3 – Process for the electronic submission of medicinal product information
- Chapter 4 – Data quality assurance
- Chapter 5 – Data access/export
- Chapter 6 – Technical specifications on structure and format: Technical specifications for the API, contains description of principles, security, resources, calls, end-points
- Chapter 7 – Migration guide: migration rules between xEVMPD and PMS including backwards compatibility rules
- Chapter 8 – Practical examples
-
Chapter 9 – Process for submitting existing data on medicinal products authorised for human use